Introduction:
Collaborative efforts in medical education that include patient organizations have been shown to be instrumental in enhancing clinical outcomes and exposing professional and patient learners to practice-changing evidence. Here we describe the educational and practical impact of a strategic partnership between PeerView Institute for Medical Education (PeerView) and CLL Society that has led to successful outcomes across six educational initiatives on management of patients with chronic lymphocytic leukemia (CLL) developed for a variety of professional audiences.
Methods:
Six educational initiatives targeted healthcare professionals, including hematologist-oncologists, oncology nurses, pharmacists, and other relevant professionals, involved in the management of patients with CLL; patients with CLL also engaged in these activities, which included in-person and virtual live satellite symposia, in-person and virtual live regional workshops, and online self-study activities. Influenced by the partner's participation and accessibility of its patient outreach resources, the instructional design of the interventions was chosen for its ability to deliver skills-based education and comprised case-based content, concise scientific lectures, and expert guidance on using CLL Society tools/resources to engage with patients and encourage them to participate in their own care. Designed to meet the needs of the target audience, the learning objectives addressed knowledge of the science supporting the use of targeted therapies, such as BTKi and BCL2i platforms, in the CLL setting, as well as ability to manage safety concerns, personalize treatment plans, and counsel patients.
Furthermore, each initiative emphasized the patient voice through the integration of patient testimonials developed in collaboration with CLL Society or sharing of existing partner tools and resources (eg, Expert Access™ program, Patient Education ToolKIT, Test Before Treat™ biomarker educational program). These resources were made available for download to aid in retention and facilitate implementation of the education into practice, helping providers improve their ability to work in a collaborative, patient-centric manner.
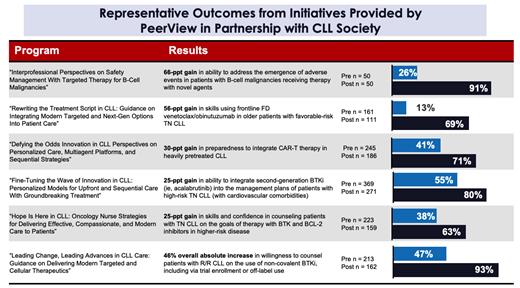
Outcomes measurements included pre- and post-assessment questions designed to quantify the impact of the educational content on learners' knowledge, skills, and clinical practice intentions. Results of these assessments were compared with baseline audience findings to determine the effect of the education.
Results:
To date, these activities have actively engaged 16,855 learners.* Pre- to post-participation score changes across all initiatives demonstrated gains of up to 69% in knowledge and 68% in skills and strategies over baseline. These substantial gains were consistent across all initiatives, highlighting the effectiveness of the educational content. Notable improvements in knowledge and skills were seen in treatment selection, identification and management of safety considerations, and ability to counsel patients with CLL. Additionally, these data demonstrated an increase in the participants' ability to incorporate evidence-based protocols and creation of personalized treatment plans into practice. Hundreds of learners, including patients with CLL, have downloaded the provided tools and resources.
*Outcomes data as of 7/26/2023.
Conclusions:
As shown with these outcomes, the initiatives led to significant improvements in healthcare professionals' knowledge, skills, and intentions regarding CLL patient care and enabled participants to achieve their specific learning objectives and make positive behavior changes that can lead to improved patient outcomes. Furthermore, the outcomes from this collaboration between PeerView and CLL Society demonstrated that incorporation of patient perspectives and tactics proven to enhance gains in knowledge and skills into medical education increases the ability of participants to effectively manage patients with CLL. Overall, these data suggest that collaborative educational initiatives with patient support groups enhance healthcare professionals' ability to deliver effective, compassionate, and modern CLL care, resulting in a positive impact on the lives of patients.
Disclosures
Koffman:MEI Pharma: Current equity holder in publicly-traded company; Bristol Myer Squibb: Current equity holder in publicly-traded company, Honoraria; Astra Zeneca: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation ; AbbVie: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation; BeiGene: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Gilead: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation; Johnson & Johnson: Current equity holder in publicly-traded company, Honoraria; Merck: Current equity holder in publicly-traded company; Miragen Therapeutics: Current equity holder in publicly-traded company; Novartis: Membership on an entity's Board of Directors or advisory committees; Portola Pharma: Current equity holder in publicly-traded company; Regeneron: Current equity holder in publicly-traded company; Sunesis Pharmaceuticals: Current equity holder in publicly-traded company; TG Therapeutics: Current equity holder in publicly-traded company. Battiato:AbbVie: Consultancy; BeiGene, Inc.: Consultancy. Gribben:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Speakers Bureau; Janssen Pharmaceuticals, Inc: Consultancy, Research Funding, Speakers Bureau; Kite, A Gilead Company: Consultancy, Speakers Bureau; Novartis: Consultancy; Bristol Myers Squibb: Speakers Bureau. Lamanna:BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Oncternal: Research Funding; Octapharma: Research Funding; Janssen: Consultancy; Pharmacyclics: Consultancy; Genentech: Consultancy, Research Funding; MingSight: Research Funding; Eli Lilly/Loxo: Research Funding; Adaptive Biotechnologies: Consultancy; TG Therapeutics: Research Funding. Russomanno:AbbVie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal